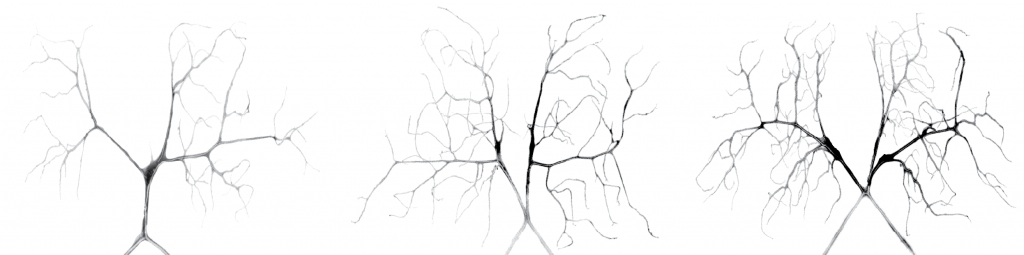
Dorsal terminal cells of Drosophila trachea are typically present in pairs (middle). Mutations in the FGF-ERK signaling pathway result in loss (left) and gain (right) of these cells.
Dynamics of living tissuesOur research aims to establish quantitative understanding of dynamic interactions of genomic instructions and physical processes in organism development. We have been dissecting these interactions in a range of developmental contexts, including embryonic metabolism and tissue patterning, growth, and morphogenesis. |
|
Quantitative biology of developmental defects
Developmental disorders are a leading cause of childhood mortality and a major factor in the lives of affected individuals and families. Focusing on disorders caused by genetically deregulated cell signaling, we are advancing quantitative biology of developmental defects using gene editing and imaging approaches in Drosophila. Our recent results in this area include the demonstration of a stochastic contribution to incomplete penetrance in monogenic disorders and a computer vision strategy for high-throughput recording and analysis of individual developmental trajectories which provide unique insights into genetic and environmental regulation of adult-to-juvenile transitions.
Dynamics in small cell clusters
The formation of animal egg and sperm cells commonly proceeds though an evolutionarily conserved cyst stage, where several cells are joined by cytoplasmic bridges. Small cell clusters also naturally arise during early embryogenesis, when the embryonic cleavages subdivide the fertilized egg into progressively smaller cells. We are investigating how the structure and function of such small cell clusters emerge from collective dynamics of the underlying cell division events. Our recent results in this area include the discovery of synchrony-preserving mechanism in Drosophila embryos and characterization of bidirectional cell communication processes in Drosophila oogenesis.

